[ Envirowatchers ] [ Main Menu ]
19106
From: pamela, [DNS_Address]
Subject: Trillions of Tons of Carbon Are Missing From Climate Models
|
my comments: wtf, and huh? Trillions of Tons of Carbon Are Missing From Climate Models Not ideal. BY DARREN ORF PUBLISHED: APR 22, 2024 8:00 AM EDT bookmarksSAVE ARTICLE concept illustration global warming around the world is about to be burned by human hands 3d image Surasak Suwanmake//Getty Images While the world’s soils are home to lots of organic carbon—such as leaf litter and animal waste—inorganic carbon, which is often in the form of solid carbonates, can also leak into the atmosphere. And it isn’t accounted for by current climate models. A new study focuses on the role of soils as both a storage for and emitter of carbon, and found that 23 billion tonnes of inorganic carbon could escape soil over the next 30 years. Good land management—as well as other practices, such as afforestation and improved rock weathering—can help slow down this significant source of CO2. The sole focus of people and programs combating climate change is finding ways to keep carbon out of the atmosphere. Planting trees is a big help, as their woody roots lock away carbon for decades, and companies are hard at work trying to find artificial means of sucking greenhouse gasses from the air and sequestering it underground. But in this obsession with tracking CO2 levels, one significant source of both emission and storage has been overlooked—the soil. According to a new study by the Chinese Academy of Sciences and Australia’s Commonwealth Scientific and Industrial Research Organization, the top two meters of soil beneath our feet currently hold roughly 2.3 trillion tonnes of inorganic carbon—five times more than all of the terrestrial plants on Earth combined. RELATED STORY hypergiant industies This Machine Uses Algae to Eat Carbon Dioxide Scientists arrived at this number by analyzing 200,000 soil samples from around the world, and found that concentrations of inorganic carbon were higher in arid and semi-arid landscapes where water is less likely to carry away these carbonates. While countries like Australia are particularly filled with inorganic carbon —the continent is the fifth largest repository, according to the study—its also found in wetter regions along rivers and around lakes and coastal areas. So, these carbon-locking soils impact the entire world. The results of the study were published last week in the journal Science. “This huge pool of carbon is affected by changes in the environment, especially soil acidification. Acids dissolve calcium carbonate, meaning the carbon dissolves in water or is released as carbon dioxide gas,” the researchers wrote in an article for The Conversation. “This huge pool of carbon is affected by changes in the environment, especially soil acidification. Acids dissolve calcium carbonate, meaning the carbon dissolves in water or is released as carbon dioxide gas.” RELATED STORY aerial view of office building in cloud Skyscraper Turns Carbon Dioxide Into Concrete Inorganic carbon—mostly in the form of solid carbonate minerals like limestone, marble, or chalk—is different from organic carbon like plant litter, bacteria, and animal waste. While the latter has been gaining global attention, inorganic carbon has been largely ignored as a significant tool in the Earth’s process of regulating CO2 in the atmosphere and a potential source of the climate change-inducing gas. The study estimates that some 23 billion tonnes of inorganic carbon could be released over the next 30 years, with little knowledge on how this will impact the planet’s land, water, and atmosphere. By comparison, the airline industry emits roughly 1 billion tonnes of CO2 every year, so this inorganic carbon is a not-so-insignificant amount. The study’s authors point to the importance of land practices—whether irrigation or fertilization—as well as strategies like improved rock weathering and afforestation can help keep inorganic carbon locked in soils. After all, less CO2 is the name of the climate change game, and the world’s soil has a big part to play. Headshot of Darren Orf DARREN ORF Darren lives in Portland, has a cat, and writes/edits about sci-fi and how our world works. You can find his previous stuff at Gizmodo and Paste if you look hard enough. WATCH NEXT |
19109
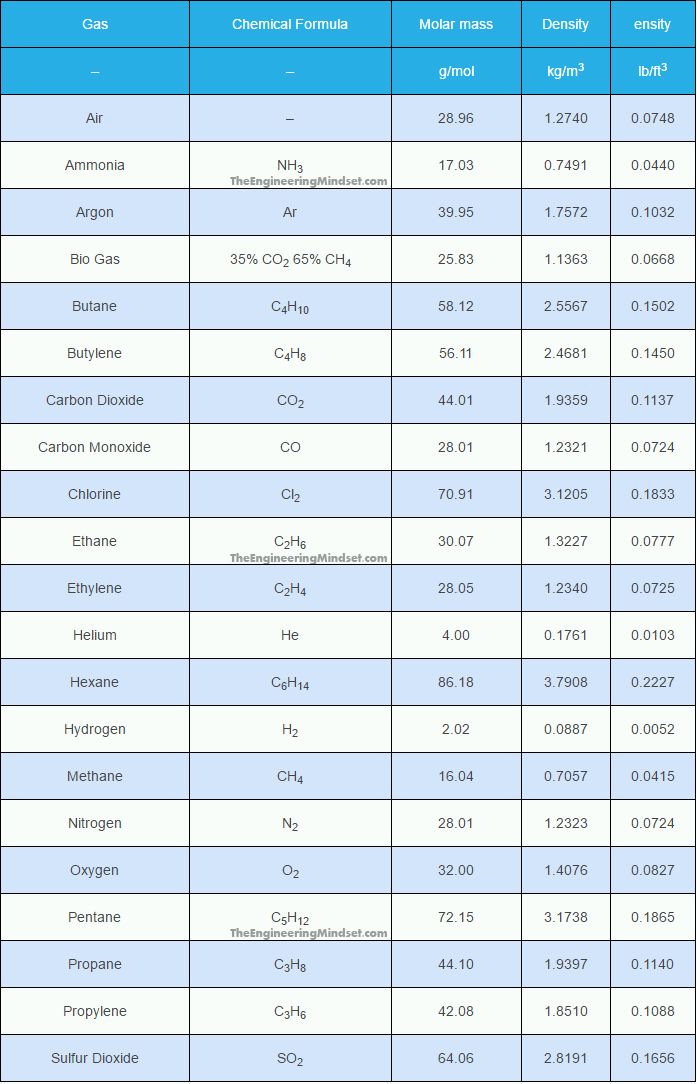
From: Jeff/Lake Almanor,CA, [DNS_Address]
Subject: CO2 is heavier then air
|
The opposite of Hydrogen and Helium. So it falls to the ground. Wishing you well.... |
19116
From: pamela, [DNS_Address]
Subject: Re: CO2 is heavier then air
| Thanks Jeff- wishing you well also |
Responses:
None
19110

From: ryan, [DNS_Address]
Subject: Re: CO2 is heavier then air
URL: https://news.climate.columbia.edu/2020/09/23/carbon-dioxide-distribution-atmosphere/
|
A handmade drawing explaining the different forces at work in a wine bottle versus the Earth’s atmosphere. Image: Ángel Muñoz Q: How does CO2 get high up into the atmosphere? With a specific gravity of about 1.5, it should fall to the earth as it cools when rising. The airlines tell us that it is -40 degrees at 30,000 ft. So why or how is it that some CO2 works its way up into the upper atmosphere? A: angel munoz in a vineyard Ángel Muñoz is an associate research scientist at the International Research Institute for Climate and Society and leads the Latin American component of the Columbia World Project “Adapting Agriculture to Climate Today, for Tomorrow” (ACToday). He is also a devoted wine connoisseur and is pictured here with Carménère grapevines in Chile. Carbon dioxide is a gas. The density of a gas increases as temperatures get colder. So, because temperatures decrease as we reach higher altitudes, gases become denser at higher altitudes. Denser objects tend to sink, pulled down by gravity. (In fact, the force of gravity pulling gas molecules towards the Earth’s surface is what maintains our atmosphere.) Different gases also have different molecular weights. CO2 is heavier than oxygen, so we might expect every CO2 molecule to sink below a layer of oxygen molecules. Generalizing this idea to the other gases in the air, we might deduce that this would result in a perfectly stratified atmosphere with separate layers of each type of gas. We can see an example of a stratified atmosphere inside a bottle of wine. When the bottle is sealed, air between the surface of the wine and bottom of the cork includes both oxygen and CO2. Because CO2 is heavier than oxygen, gravity forces CO2 molecules to form a “layer” beneath the oxygen molecules, helping to separate the wine from the oxygen. Desirable properties of wine, such taste and smell, begin to change once the liquid is fully exposed to oxygen. Without the stratification inside the sealed bottle, we wouldn’t have that cushion of CO2 to protect the wine from oxygen, giving unopened wine a much shorter shelf life or even turning it into vinegar over time. Earth’s atmosphere is not like the air inside a sealed wine bottle. Atmospheric gases are well-mixed, not stratified. This due to the force of diffusion. Gas molecules want to move, and they will expand to fill the volume within which they are contained. Confined to a tightly sealed container such as a corked wine bottle at constant temperature of about 52-57 degrees F, gasses have no room or enough “excitement” to expand and move around. They settle into layers based mostly on their molecular weights. However, the Earth’s atmosphere is much more expansive than a wine bottle. CO2 does not break down until about 80 kilometers from the Earth’s surface, giving atmospheric gases a huge expanse to occupy. Excited by the heat radiating from the Sun into the atmosphere, molecules move rapidly. As they bang into each other (for example, at 63 degrees F, CO2 molecules crash together about 7 billion times per second), the gas molecules intermingle, rather than settling in stratified layers. It is mainly diffusion that allows CO2 to integrate at altitudes higher than what its molecular weight alone would suggest, although other processes, like strong updraft and downdraft air currents, are also involved. drawing of co2 behavior Similarly, upon uncorking that wine bottle for the first time and bringing it from the cellar to a warmer room temperature, the trapped gasses become a part of the larger atmosphere. Gas molecules mix, and after the bottle’s vacuum seal has been broken, replacing the cork means well-mixed molecules remain in the bottle once you replace the cork yourself. Oxygen is now able to reach the wine, eventually causing the wine to taste “off.” Anyone who has opened a bottle of wine to “let it breathe” before drinking it knows that some amount of oxygenation can improve the wine’s taste, but eventually oxygenation will ruin those desirable qualities. So, remember to responsibly consume a bottle of wine within a few days for best flavor. And remember that even in an airplane at 30,000 feet, gas molecules in an open bottle of wine will mix just as they do in the rest of atmosphere! |
19111

From: Jeff/Lake Almanor,CA, [DNS_Address]
Subject: Parts of our Atmosphere
URL: https://education.nationalgeographic.org/resource/parts-atmosphere/
|
Very interesting. As the upper parts of our atmosphere is stratified. But every layer is described in detail, and what each layer accommodates. The thermosphere is the thickest layer in the atmosphere. Only the lightest gases—mostly oxygen, helium, and hydrogen—are found here. But this article complements your article / post, and deserves a nice glass of fine wine while digesting. 2019 Domaine de la Romanee-Conti, Romanee-Conti Grand Cru 3x750ml, A three bottle case for $150.000.00 |
19113
From: ryan, [DNS_Address]
Subject: Re: Parts of our Atmosphere
| believe what you want to...you will anyway...lol... |
Responses:
[19115]
19115
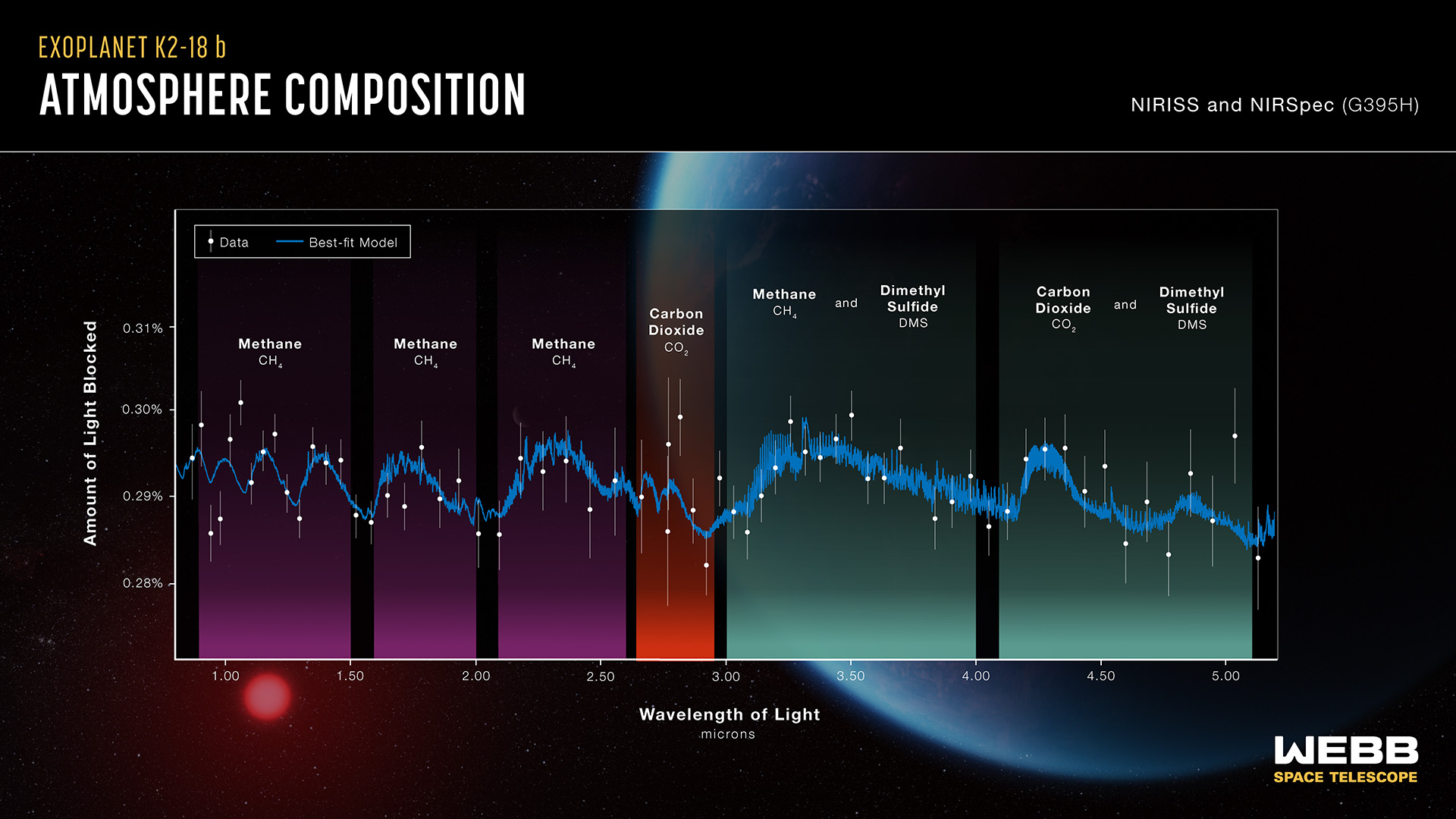
From: Jeff/Lake Almanor,CA, [DNS_Address]
Subject: Webb Discovers Methane, Carbon Dioxide in Atmosphere of K2-18 b
|
Well what do you know about that... We must have found life on a distant planet, only 120 light years from Earth. Or could be only an extinct population, due to misusing fossil fuels, and raising livestock. But the Dimethyl Sulfide show a global water ocean. If nothing else, I'm learning new stuff. That includes your first reply, but the second one is a given. LOL |
Responses:
None
[ Envirowatchers ] [ Main Menu ]